In Chemistry, what is the Periodic Law?
 Jessica Ellis
Jessica Ellis
The periodic law is one of the foundations of chemistry. The law suggests that elements, when arranged by atomic weight, tend to have similar characteristics at certain intervals from one another. Credit for the formalization of the periodic law nearly always goes to Dmitri Mendeleyev, a Russian chemist. In truth, the discovery was the result of nearly a century of frantic work of a variety of scientists, all obsessed with determining the properties and even discovering their very own elements. The modern periodic table, a staple in every school science room, is actually a repeatedly refined and reorganized version of Mendeleyev's original chart.
During the 18th and 19th century, a new element appeared to be popping up every week. With advanced methods of chemistry allowing better examination of minute substances, the pursuit of the elements became a never-ending hunt for many of the greatest scientists of the day. With such a profusion of elements being discovered and described, it soon became the preoccupation of many to organize the elements into a list that made some kind of rational sense.
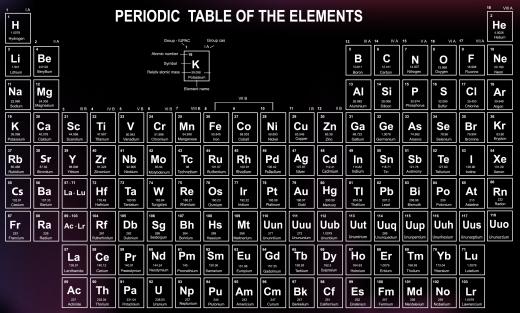
Elements are primarily described by a few defining features: the number of protons in the nucleus, from which the atomic number is derived, calculations of the mass that define the atomic weight, and behavior. Many different attempts were made to arrange the elements so that any of these factors lined up in a sensible fashion, but like a moving puzzle, every time one piece was put in order, the others became disordered. The periodic law, a theory that would line up disparate information into a tidy table, seemed out of reach.
Though Mendeleyev rightly deserves credit for the modern periodic table and the pulling together of all the threads that form the periodic law, he was not the first to try, by any means. John Newlands, an English chemist, noted the tendency of elements to have similar behavior when lined up by atomic weight; notably, that every 8 intervals, an uncanny similarity appeared. His “theory of octaves” likened elements to keys on a piano, where every eight keys forms a repeating set. A French scientist, Alexandre-Emile Béguyer de Chancourtois, also noted the repeating properties and devised a table that arranged elements in a helix shape. The work of both men was largely ignored by the scientific community, and Newlands was often ridiculed for his comparison.
Mendeleyev's table illustrated the periodic law at a glance by lining the elements up horizontally by atomic weight and vertically by similar properties. Thus the alkali metals of lithium, sodium, potassium, rubidium, caeseum, and francium make an orderly row down the left side of the table, all while remaining in order by atomic weight. Since not all elements were discovered at the time of the tables formation, Mendeleyev simply left spaces in the table for those elements that should fit in, according to his theory.
The periodic law gave insight into a system of organization within chemistry that had previously only been suspected. By turning the organization of elements into a neat table by using the periodic law, Mendeleyev made it obvious at a glance which elements shared certain properties. Though the table was later remodeled and reorganized by the British physicist John Moseley, the inferences and theory of Mendeleyev remain largely unchallenged more than a century after his death.
AS FEATURED ON:
AS FEATURED ON:











Discuss this Article
Post your comments