What are Biopharmaceuticals?
 Mary McMahon
Mary McMahon
Biopharmaceuticals are drugs which are produced with the means of biotechnology. There are a number of ways in which such drugs can be made, but the key distinction between them and other drugs is that they are not extracted from a native source or synthesized with chemical reactions. Instead, they are created with the use of living organisms which may have been modified to produce the desired compound. This requires the use of specialized equipment and clean rooms for safety which protect the integrity of the pharmaceutical compounds while they are produced and packaged.
One classic method of making biopharmaceuticals involves the use of a bioreactor, a container which is used to create tightly controlled conditions which facilitate the growth of a particular organism. In a bioreactor, drugs can be produced by organisms which generate biopharmaceuticals as a byproduct of their life cyle, often because these organisms have been modified to produce specific proteins and nucleic acids. Cell cultures and modified microbes can both be used in bioreactors to make drugs and compounds which can be used in the production of pharmaceuticals.
Genetic modification of plants and animals may also be used to make biopharmaceuticals. Transgenic cows may be designed, for example, to secrete a specific compound in their milk. The practice of using transgenic organisms for the production of pharmaceuticals has been controversial in some regions of the world, for reasons varying from ethical concerns to worries that such organisms could cross-breed with conventional organisms and become contaminated.

A variety of substances can be made using biopharmaceutical techniques, including blood factors, interferons, hormones, vaccines, and monoclonal antibodies. When researchers develop new biopharmaceuticals, they typically file for patents to protect their inventions and the process, and go through a series of steps to seek approval so that their drugs can be sold on the open market. These steps involve extensive testing for safety and efficacy, to confirm that the drugs work as claimed.
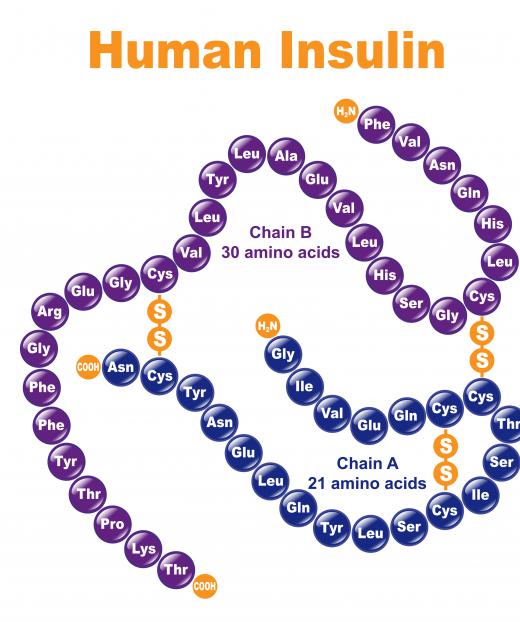
The first biopharmaceutical to hit the market was artificial human insulin, which was released in 1982 for use by diabetics. The biopharmaceuticals industry exploded after the 1980s, thanks to increasing interest in additional medical treatments, and advancements in laboratory science which made new developments possible. One advantage to such drugs, especially as an alternative to native compounds, is that they tend to be safer and the dosing is extremely reliable, because the production conditions are very tightly controlled.
AS FEATURED ON:
AS FEATURED ON:













Discussion Comments
@behaviourism- Yes, some advances have been great, I admit though that I am one of those who worries about this stuff. It seems like we have many medicines these days that do nothing or treat a "problem" that really isn't one.
I know some people are worried about when biopharmaceuticals will "stop" evolving, because chemicals might become too dangerous, but there are so many advances that have been made. I love the idea of artificial "human" insulin in particular; it means that people no longer have to rely solely on animal insulin for human diabetes, making us more independent.
Post your comments