What are Thermodynamic Properties?
In science, thermodynamic properties are characteristics used to describe a physical system. They refer to qualities like heat, pressure, and temperature, which affect phenomena from the Earth's atmosphere to the rates at which chemical reactions occur. Heat exchange between objects occurs nearly everywhere in the natural world and is very important to the function of modern technology. Thermodynamic properties measure the various factors that influence this process between two or more objects. Engineers use these to design better, more efficient machines.
Thermodynamic properties refer to the parameters by which scientists and engineers analyze a particular region, called a physical system, such as an engine or a natural object. Remaining constant throughout a system, things like temperature and pressure provide information about how something uses energy and performs work. These properties are used to determine questions such as how much work a given machine can perform or the amount of energy needed to accelerate a chemical reaction in industry. They can be used to categorize a system as open or closed, according to whether both matter and energy can flow in and out of it.
The heat that must be put into a system and the work that must be done to it in order to increase its internal energy all are thermodynamic properties. Energy can be transferred by heat between objects of different temperatures. Spontaneous heat transfer occurs when heat moves from a body with a higher temperature toward a colder object, whereas the reverse movement requires work to be done. Free energy is the measurement of how much of a thermodynamic system's energy can be used to do work, whereas entropy measures the amount of energy lost, wasted, or otherwise unused.

Thermodynamic temperature is an important property because it allows scientists and engineers to calculate the absolute temperature of an object. It is a measure of a system's heat loss and absorption, which together represent the exchange of energy occurring in it. Since thermodynamics is a branch of science concerned with energy exchange and conversion, this property is essential to describe the state of a system. Properties like temperature are said to be intensive because they are independent of a given system's size, unlike volume or pressure, which vary with the object's size.
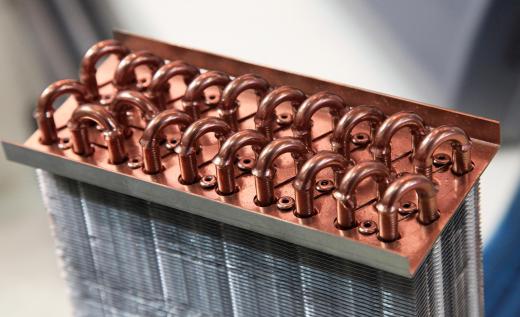
Engineers and chemists use thermodynamic properties to build engines and plan chemical reactions that maximize the efficient use of heat energy. Thermodynamic principles were discovered in part during the Industrial Revolution during the quest to make more efficient machines, particularly those in steam-driven textile plants. This early emphasis on the applied scientific use of thermodynamic properties led to many practical discoveries. An example of this information's practical value is found in the design of heat exchangers, such as car radiators, which mediate the transfer of heat energy from one object to another.
AS FEATURED ON:
AS FEATURED ON:












Discuss this Article
Post your comments