What Is a Dimer?
A dimer is a chemical compound that consists of two monomers, or subunits, which are structurally similar. Two similar molecules bonded together form a dimer, while many similar molecules bonded together would form a polymer. Dimers are commonly held together by covalent or hydrogen bonds. They are often important in the fields of biochemistry and especially medicine, where they are involved in the diagnosis of certain diseases.
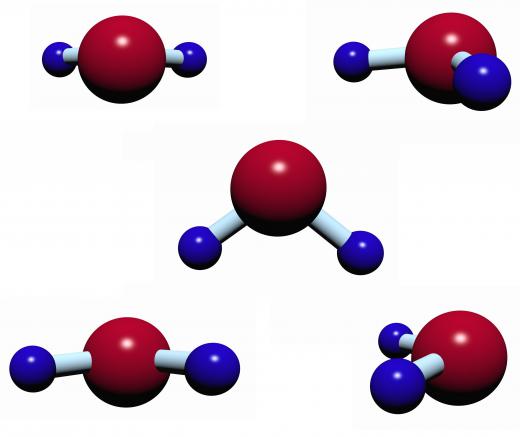
An example of a dimer that forms through hydrogen bonding is the water dimer. The water molecule, which consists of two hydrogen atoms and one oxygen atom arranged in a triangular shape, is a polar molecule — in other words, there is a separation of electric charge across its molecular structure. Electrons, which carry a negative charge, are more concentrated at the oxygen end of the molecule than at the hydrogen end. This means that the hydrogen end carries a positive charge, while the oxygen end carries a negative charge. Two water molecules connect through hydrogen bonding when the hydrogen end of one molecule is attracted to the oxygen end of the other.
Carboxylic acids are another type of chemical compound that tend to form stable dimers through hydrogen bonding. These organic acids contain one or more carboxyl groups, a molecular structure consisting of carbon, oxygen, and hydrogen. Acetic acid, which is found in vinegar, forms dimers in its crystalline and gas states. Carboxylic acids boil at higher temperatures than water because more energy is required to vaporize their stronger structures.
Dimer acids, molecules related to carboxylic acids, are important in industrial applications. These substances are created from fatty acids and may be used in adhesives, resins, lubricants, and fuel oil. The primary component of dimer acid is stearic acid, which is an organic molecule found in vegetable and animal fat and which is also sold commercially for laboratory use.
In medicine, dimers are an important tool for diagnosing thrombosis, a condition in which a blood clot inside a vein obstructs circulatory flow. The clot is built on the foundation of cross-linked fragments of protein, which then degrade to reveal an underlying structure known as the D-dimer. Elevated levels of D-dimer in the bloodstream indicate that clots are being formed, making thrombosis a likely diagnosis.
Linkage between structural units can also be a problem in deoxyribonucleic acid (DNA), the molecule containing a cell’s genetic information. DNA, which is made up of repeating subunits, is vulnerable to damage by ultraviolet (UV) light. Exposure to UV light can cause two subunits of DNA to fuse together through covalent bonds, creating a dimer. This fusion makes it impossible for the cell to correctly process the DNA, eventually resulting in mutations and skin conditions including melanoma, a dangerous skin cancer.
AS FEATURED ON:
AS FEATURED ON:











Discuss this Article
Post your comments