What Is a Nonpolar Bond?
A nonpolar bond is a covalent bond between atoms in which electrons are shared equally between the atoms. The equal sharing of electrons results in the formation of a nonpolar molecule that has no electric dipole moment, or separation of electric charge. Two identical atoms will form a nonpolar bond because they have equal electronegativities.
A common example of a nonpolar bond can be found in diatomic oxygen. Each oxygen atom has six electrons in its outer shell, needing two more to reach the stable eight-electron noble gas configuration. In the oxygen molecule, the atoms share a set of four electrons equally in double bonds, satisfying each atom’s need for an extra two electrons. Each of these bonds would be considered a nonpolar bond.
Nonpolar covalent bonds tend to be found in diatomic molecules, where two identical atoms are bonded together. These include iodine, hydrogen, and nitrogen. The overall polarity of a molecule should not be confused with the polarity of its bonds. It is possible for a molecule to be nonpolar as a whole, even when its atoms are not linked through a nonpolar bond. This occurs when polar covalent bonds cancel each other's charge due to the molecular structure.

In methane, carbon is bonded to hydrogen in bonds that are slightly polar, involving somewhat unequal sharing of electrons. The tetrahedral structure of the molecule causes these charges to cancel out, resulting in a molecule that is nonpolar. Even though the atoms are not bonded through nonpolar bonds, the molecule behaves in a nonpolar way.
This overall nonpolar interaction between hydrogen and carbon atoms makes organic compounds hydrophobic, meaning that they cannot interact with water to form hydrogen bonds. When interacting with polar molecules, water forms hydrogen bonds between its own positively charged hydrogen atoms and an electronegative atom from another molecule. Nonpolar compounds cannot perform this interaction because they have no separation of charge across their structure, and thus no site to attract a charge.
Hydrophobic behavior can be observed in household products such as vegetable oil, which separates from water visibly. The nonpolarity of hydrophobic substances is also an important factor in the functioning of living organisms. Lipids, which occur in cell structures, prevent water from mixing with internal structures and separate fluids. As with other organic compounds, these molecules consist of bonds that are almost, but not quite, non-polar: their bond structure causes their polarity to cancel out.
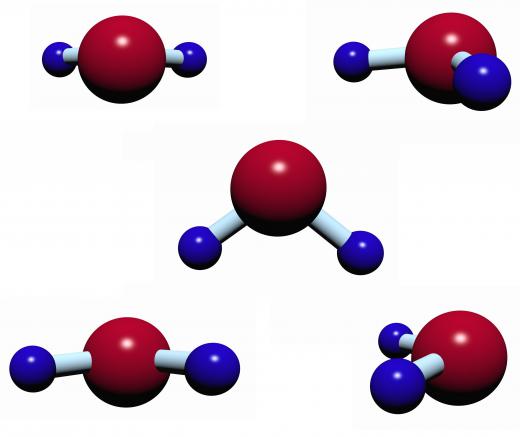
Carbon dioxide is another example of a nonpolar molecule with polar bonds. The structure of this molecule is linear, with two oxygen atoms double-bonded to a central carbon atom. These bonds are polar covalent, but because they are exactly symmetrical, their charges cancel out, creating a nonpolar molecule.
AS FEATURED ON:
AS FEATURED ON:












Discuss this Article
Post your comments