What is Atomic Theory?
 Michael Anissimov
Michael Anissimov
Atomic theory is the idea that matter is made up of little units called atoms. When the ancient Greek philosopher Democritus came up with the idea in the 5th century BC, is was originally meant to refer to indivisible units. As of 1897, the British scientist J.J. Thomson discovered that atoms are in fact made up of smaller particles. Today, this theory refers to matter being made up of units that are indivisible only some of the time. Exceptions include plasmas, such as fire, other ionic arrangements, such as those found in the body, radioactive materials, and many more.
Even though atomic theory today is a familiar cornerstone of modern science, like germ theory or evolution, throughout most of human history, people believed that matter was probably continuous and could be broken down into arbitrarily small quantities. It wasn't until 1803, or possibly a bit before, that the English chemist John Dalton revived the old idea and used it to solve various problems that chemists were grappling with at the time. Rather than any one experiment leading to the idea, it emerged from analysis of multiple experiments involving the properties of gases and chemical reactions. His theory was popularized and confirmed experimentally over the course of the early 19th century.
Dalton's atomic theory had five main points:
- All matter consists of minuscule particles called atoms.
- All atoms of a given element are identical to each other.
- All atoms of a given element are different than those of other elements.
- Atoms of one element combine with other elements to create compounds. They always combine in equal amounts.
- Atoms cannot be created, divided, nor destroyed.
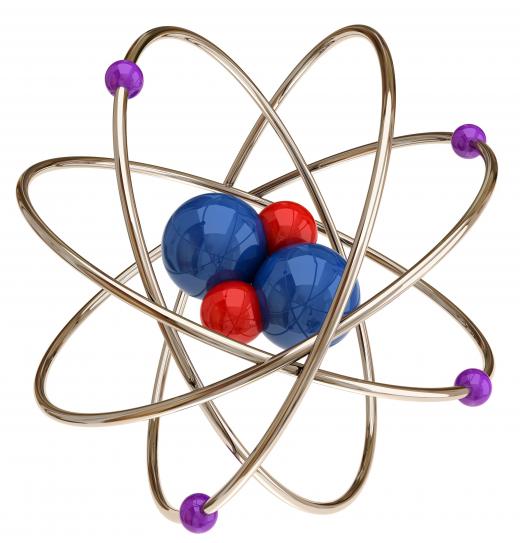
Most of the above is still accepted by scientists today, except for a few points. First, the discovery of nuclear fusion/fission and radioactivity prompted revision of point #2. Isotopes prove that atoms of the same element can actually have small differences due to a different number of neutrons. Also, the existence of ions with varying numbers of electrons also contradicts this point.
The fifth point is also invalidated by nuclear physics, since atoms can indeed by destroyed in nuclear chain reactions. The second item of point #4 is also quite incorrect, as, for instance, water is H2O, not HO. His insistence that atoms combine in equal amounts to create compounds held back acceptance of his theory for years. Regardless, from the viewpoint of today, Dalton contributed remarkably for his time, and his name continues to be revered by its association with the theory.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
I thought that a nucleus is an atom.
@pastanaga - The idea that atoms are the smallest building blocks in creation is definitely not taken for granted, and in fact, they have discovered many other particle types which are smaller than atoms (although I don't think they make up atoms, they just exist alongside them).
I don't really understand physics when it gets that theoretical though, it seems to get very complicated and slightly wishy-washy but I'm sure it makes sense to those who are studying it.
I think it's interesting to speculate that we may still have not found the lower limit of size for matter. Atoms might be made up of even smaller particles, there's no reason why they can't be.
I guess I kind of like the idea that size differences can go on forever, getting smaller and smaller or bigger and bigger so that space is infinite in more directions than we might think.
@anon112649 - Unfortunately a lot of the time science history tends to be a bit Western-centric, probably because when it is in English it's being written by a Westerner who might not know about other theories.
In this case though, I think it's talking about a specific kind of modern atomic theory, which is attributed to a particular person. If they were talking about the history of atoms in science, they'd have to mention a lot of other people, since the idea of tiny particles has been around for a while in both the East and the West.
What about Bohr? What part did he have in it?
Were there any problems with this theory?
Name five areas where the atomic theory is used.
What about the second point, though?
"2) All atoms of a given element have are identical to each other."
Aren't isotopes the same given element but different amount of nuclei? More neutrons?
What a delightfully succinct post.
this was short and to the point, also very understanding, thank you it was a big help and makes studying easier because of the information given.
Thanks! perfect for my project!
good site
thank you so much for this site. it got down right to the point, and had exactly everything i needed.
this site was to the point and precise. wisegeek
is the best!
This site is very helpful for my project but i felt very sorry to say that our indian philosopher rushi kanaad's reference is not in this page. - vibha s.
thank you so much! This was very helpful, and i also decided who to do my project on. I had to figure out what atomic theory was before i could do the project on it.
thanks for helping me to understand the atomic theory. now i know!
thank you very much.
thanks very much for this website. It has helped me and my partner to complete our task! thanks again.
this website helped me a lot in science class!
Thanks.
Thanks. you helped a lot.
This site was great! it helped me actually understand atomic theory and helped a lot on my project. thanks!
this site was all i needed for my project. thanks!
This site was very helpful for my project. thank you to the wisegeek creator!
Post your comments