What is Binding Energy?
Binding energy is the energy needed to remove a particle from an atom. Each part of an atom has binding energy, but the term is commonly used to refer to the energy required to split the nucleus of an atom. This energy is integral to discussions of nuclear fission and fusion. Electron binding energy is more commonly called ionization energy.
The energy in nuclear bonds may be observed by measuring an atom's mass, which is less than the sum of its components' mass. This is because some of the mass of the nuclear particles is converted into energy according to the equation E=mc2. The missing mass is the source of the binding energy. The smallest atoms have the lowest nuclear binding energy. It tends to increase with atomic number up to iron, which has the highest binding energy; larger atoms are more unstable.
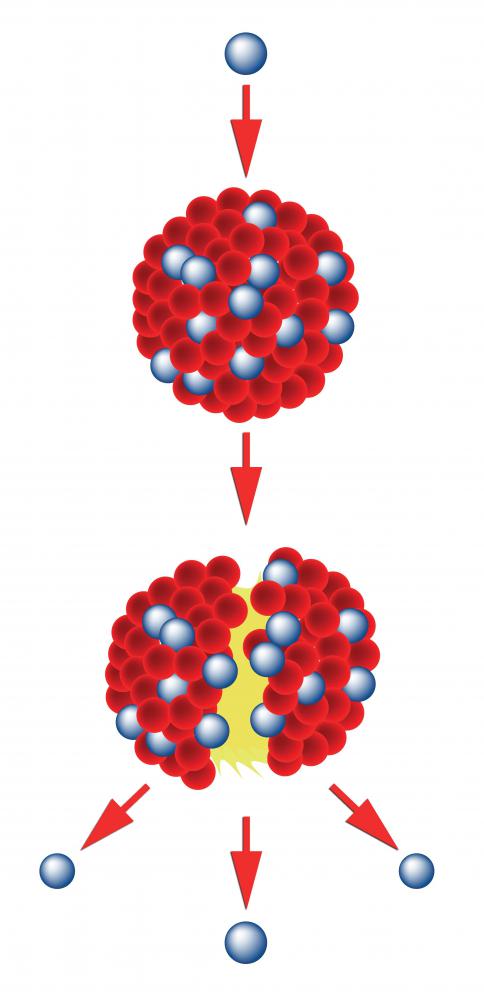
Nuclei are made of protons and neutrons. Similar charges repel. Protons are positively charged, and neutrons, which are neutral, provide no balancing negative charge. The bonds of the nucleus must be strong enough to overcome the repellent forces of the positive charges on the protons. Consequently, there is a large quantity of energy stored in those bonds.
The processes of nuclear fission and fusion rely on the release of nuclear binding energy. In fusion, deuterium, a hydrogen atom with one neutron, and tritium, a hydrogen atom with two neutrons, bond to form a helium atom and a spare neutron. The reaction releases energy equal to the difference between the binding energy before and after the fusion. In fission, a large atom, like uranium, splits into smaller atoms. The decomposing nucleus releases neutron radiation and large amounts of energy from the changing strength of nuclear bonds in the new atoms.
The ionization energy of an electron varies based on the type of atom from which it is being separated and the number of electrons that have been removed from that atom before. Removing outer electrons requires less energy than removing inner ones, and more energy is needed to split up a pair than to remove a lone electron. The difference in ionization energies is the reason that some configurations are more stable than others: the higher the next ionization energy, the more stable is the state of the atom. Stable compounds dominate in nature; ionization energies literally shape the world.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
@MrMoody - I think you’re out on a very big limb.
It is interesting speculation, but I think there’s more to the missing mass observation about the universe than the superficial similarity with masses missing in atoms.
If your theory were correct, the universe itself could be split to create mind bending atomic explosions.
My understanding from the article is that binding energy is energy that is lost, not energy that is truly invisible or never was there to begin with. Therefore I don't think you can draw a connection between this and the missing mass in the universe, which scientists admit they can't even observe.
I do think you raise an interesting idea, however.
So there is a mass defect or missing mass in all atomic particles. I think I understand the basic concept, but it triggers another idea that I’ve heard a lot about in science.
It’s the idea of missing mass in the universe. Scientists like to point out that most of the universe is missing mass.
While I don’t fully understand the implications of this, could there be a connection between this and the missing mass in every atom? Maybe the universe is one big cosmic atom, and the missing mass is its collective mass defect.
I realize I’m out on a limb here, but it’s just a thought.
Post your comments