What is Capillary Action?
 Mary McMahon
Mary McMahon
Capillary action is a principle that explains why fluids are often drawn up into other substances. This phenomenon is also sometimes described as “capillarity.” A classic example of this action involves a paper towel and a spilled puddle of water: when the towel is dipped into the water, it sucks the water up. It explains a large number of events that occur in nature, from how trees manage to get water all the way up to their crowns to the way in which water seems to climb up a straw.
Several factors are involved in capillary action. The first is cohesion, the tendency of molecules of a substance to stick together. Water is a cohesive element, with a level of cohesion that creates a high degree of surface tension. When water is spilled on a table, it tends to stick together in a puddle, rather than spreading out, because it is cohesive.

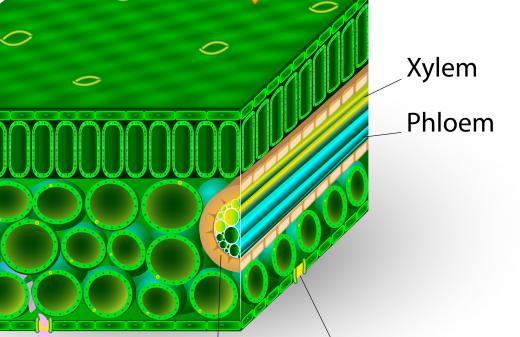
The second factor is adhesion, the tendency of some substances to be drawn to unlike substances. In the example of a tree and the water in the ground, the liquid is drawn to the cellulose fibers in the tree trunk, which form small capillaries known as xylem. As the fluid adheres, it creates a meniscus, a small curve, along the edges of the xylem. The surface tension in the water causes the water to climb up as the meniscus forms, because of the adhesion force between the wood and water molecules, and a new meniscus will form as the water is drawn further up into the tree. Without any effort on its part, the tree can draw the water all the way up into its top branches.
When a meniscus curves down, creating a concave surface, the fluid is said to be “wetting” the substance it is drawn to, creating the circumstances necessary for capillary action to occur. For a simple example of wetting, fill a glass of water and note the shape of the meniscus. It should be higher at the sides of the glass, with the surface of the water in the middle of the glass being notably lower. When a convex surface forms, the liquid is not wetting the surface, because the liquid's cohesion is stronger than the adhesive forces that promote capillarity. Mercury is an example of a nonwetting liquid.
The denser a liquid is, the less likely it is to demonstrate capillarity. It is also less common with liquids that have a very high level of cohesion, because the individual molecules in the fluid are drawn more tightly to each other than they are to an opposing surface. Eventually, capillary action will also reach a balance point, in which the forces of adhesion and cohesion are equal, and the weight of the liquid holds it in place. As a general rule, the smaller the tube, the higher up it the fluid will be drawn.
AS FEATURED ON:
AS FEATURED ON:













Discussion Comments
Thank you so much for writing this! I have a chem unit test tomorrow, and I was only recently introduced to capillary action. Finding a website post that sufficiently answered all my questions in a way that was easy to understand was not a simple task. Very informative and thorough explanation - thanks!
Your capillary action description is informative. Like your article states, there is a limit to the height capillary action can work with water. My high school biology teacher said capillary action in xylem works to about 22 feet, with transpiration pull being effective to about 75 feet. I could be wrong about the TP number, but it was much less than the tallest trees. Is there a theory on how the tallest trees get water to their top leaves?
Post your comments