What is Dysprosium?
 Mary McMahon
Mary McMahon
Dysprosium is a metallic chemical element in the lanthanide group of the periodic table of elements; elements in this group are sometimes referred to as rare earth metals. The element is primarily used in research and industrial applications, and most consumers do not handle it directly, although they may benefit from its use or purchase products which are made with the assistance of dysprosium. The element almost never occurs in a pure form in nature; it is extracted from a variety of minerals, and world's most major producer of dysprosium is China. The element tends to be relatively expensive, since it is difficult to extract reliably.
When dysprosium is purified, it is a very soft, silvery metal which can be cut with a knife or shears. At room temperature, the element is reasonably stable, although it will start to oxidize at higher temperatures. The metal can also demonstrate differing magnetic properties, depending on ambient temperatures. You can find dysprosium on the periodic table of elements by looking for the symbol Dy, and the element has an atomic number of 66.
Credit for discovery of the element is given to Paul Emile Lecoq de Boisbaudran, who was able to prove that the element existed in 1886, although he could not isolate it. The element proved extremely difficult to isolate, leading scientists to name it after the Greek dysprositos, which means “difficult to approach.” It took another 80 years to learn to extract this element, and it is still challenging to isolate today.
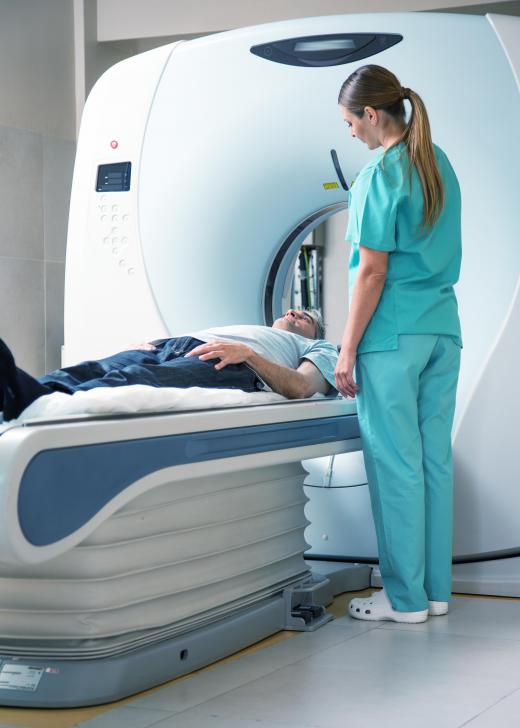
The element is often used in the manufacturing of compact discs, and it is also used as a dopant in lasers and semiconductors. Dysprosium's unique magnetic properties are utilized in magnetic resonance imaging machines for the purpose of medical imaging studies, and the element is also used in nuclear research. Some nuclear reactors have dysprosium alloys in their nuclear control rods, designed to safely bring reactor temperatures down.
Like other rare earth elements, dysprosium does not appear to have any biological use, and it is believed to be slightly toxic. Various dysprosium compounds and isotopes are certainly toxic, and they usually come with handling warnings. General precautions like face and eye protections should be used when working with the element, and it is a good idea to avoid ingesting it or handling it with bare skin.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
This is a complex, rare mineral. The uses of dysprosium are limited. One of the most common uses for the element is as a coolant for nuclear reactor rods. It is a very shiny metal, but it is soft enough to be sawn apart fairly easily. Dysprosium is sometimes used in laser materials, when mixed with other rare elements like vanadium.
Dysprosium is never found in nature, at least not as a free element. Chemistry students should know thatDysprosium's atomic number is 66 and the symbol is Dy.
Post your comments