What is Ethanol Combustion?
Combustion is a term that simply means the burning of things. It happens all of the time around us, from the flame of a lighter to the controlled explosion that powers a muscle car’s engine. Different substances for combustion are constantly being considered for various reasons, including the abundance of the substance being burned and what is produced when that happens. One such substance often used for combustion is ethanol, also known as ethyl alcohol or grain alcohol.
Ethanol combustion is fairly simple. Ethanol and oxygen enter a chemical reaction with the help of a tiny bit of energy. The reaction results in a significant release of energy in the form of heat and light, as well as the formation of carbon dioxide and water.
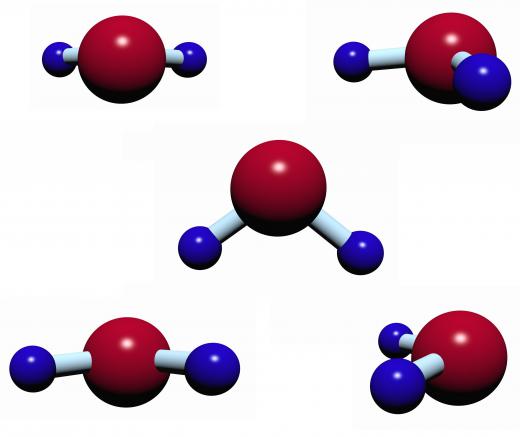
Ethanol combustion can be represented chemically using molecular formulas. Ethanol, represented by C2H5OH, combines with six oxygen atoms denoted as 3O2. When the reaction is initiated through the addition of energy in the form of heat or a spark, 2CO2 and 3H2O (two carbon dioxides and three water molecules) are formed. Energy is also released when the reaction occurs.
Ethyl alcohol has advantages and disadvantages over other common fuels. Ethanol combustion produces a quiet flame that gives off few major pollutants when compared to petroleum-based fuels. Ethyl alcohol is also relatively easily made from fermenting plant materials, while other fuels require significantly more difficult processes to create. Ethanol combustion, however, produces less heat energy than many other commonly used combustible materials.
One of the most common places where ethanol combustion occurs is in vehicle engines. It is not uncommon for some farm machines and other lightweight vehicles to be powered only by ethanol fuel. Most cars in the United States of America run on a mixture of petroleum gasoline and a small percentage of ethanol, though this requires that the vehicle’s fuel injectors be properly tunable. In Brazil and a few other countries, automobiles can be found that run on either near-pure ethyl alcohol or very high percentage mixtures.
Another common use for ethanol is in ethanol burners. These devices are also known as alcohol lamps and spirit stoves. They burn alcohol in order to produce heat and light, and can be found in science laboratories, boats, camping kits, and homes. Some ethanol burners simply use a wick to feed alcohol to the site of combustion, while others use gravity or more complex systems. These devices tend not to burn as hot as similar ones that use other fuels, but they are considered safer in many situations.
AS FEATURED ON:
AS FEATURED ON:











Discussion Comments
My mechanics say that most vehicles' engines are not equipped to deal with the upped ethanol that is being proposed. While this might seem like a viable answer to a created fuel shortage, it is very harmful for engines because of numerous flaws like vapor locking, hard starting and just operating at high temps can cause. No thank you
Living in an area where the summer temps can hit well over 100 degrees. Can you imagine the grid lock with several cars in an intersection all with vapor lock? There has to be some sort of additive that can slow down this train wreck of a none solution!
@ parmnparsley- I heard about that MIT study, and it found that the most efficient and best performing engine would be a turbo or supercharged, direct dual fuel injections system. This system would allow for the combustion of ethanol and gasoline while allowing for variable compression. The result would be an engine that is more efficient than the best hybrids or turbo diesels, and with performance characteristics that rivaled top high-octane engines.
@ glassaxe- E100 is a much cleaner burning fuel, but it has less energy per liter than gasoline. Ethanol engines can have much higher compression ratios because of this, allowing for more torque and horsepower and less of some emissions. There have been studies done by MIT that have produced ethanol fuel with octane ratings in the 130s.
As far as fuel economy goes, ethanol gets about 20-30% less fuel economy than gasoline. It is actually greater (around 50%), but with the increases in compression, economy increases. Comparably, ethanol produces about 0.5 kg/L less CO2 emissions than gasoline, but when the amount of energy per liter is taken into account gasoline becomes approximately 20% more efficient.
Gasoline is more efficient and has more energy per unit volume than ethanol, but ethanol allows for improved dynamics in a combustion motor. E100 will produce better performance than gasoline, but at the cost of efficiency and price.
What are the pros and cons of using pure ethanol versus pure gasoline? I already know it is a renewable fuel...I am talking more along the lines of combustion and efficiency.
Post your comments