What is Protein Phosphorylation?
Protein phosphorylation is the attachment of a phosphate (PO4) group to a protein. The new phosphorus group alters the role of the protein: it can activate, deactivate, or cause a change in function. Protein phosphorylation is fairly common in cells of prokaryotic and eukaryotic organisms. It provides a way for the cell to regulate biological functions without having to change the actual amount of proteins available to perform them.
A molecule called a protein kinase, sometimes called a phosphotransferase, is responsible for inducing protein phosphorylation. There are many different protein kinases, all with different target proteins. Often, the activity of a protein kinase is itself dependent on phosphorylation. This process is dependent on another kinase. Sometimes a cell uses a string of reactions, called a phosphorylation cascade, to produce an outcome. The impetus for this type of event is usually a signal from outside the cell. Usually, the energy and the phosphate group for these operations comes from adenosine triphosphate, a ubiquitous feature of the cellular landscape.
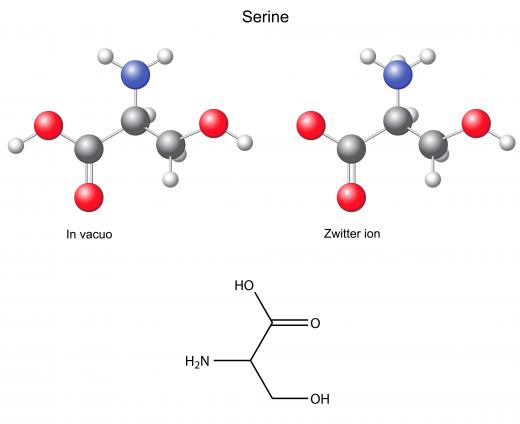
Once the new phosphate group is added by protein phosphorylation, it changes the structure of its host protein. The shape of a whole protein—called its tertiary structure—is dependent on a variety of factors, including electrical charge. The negative charge of the phosphate group changes the tertiary structure sufficiently to alter the function of the whole protein. Some proteins can be phosphorylated at multiple locations, with different effects resulting from each. Phosphorylation only occurs at specific amino acids: serine, threonine, and tyrosine.
Protein phosphorylation is a crucial element of biological homeostasis. Most cellular processes are stochastic—they rely on a set of partially random interactions that can only be managed statistically. Because most functions are carried out by proteins, the cell's usual way of performing some operation involves making more or fewer of the enzyme that carries it out. This system is relatively slow; it also is more difficult to undo, since most proteins will only stop functioning when they are destroyed.
The effects of protein phosphorylation can be undone by an enzyme called a phosphatase. This process is called dephosphorylation. Dephosphorylation works almost exactly like phosphorylation. Each process requires the other to be useful. It is the possibility of rapidly phosphorylating then dephosphorylating that makes this pathway a finer means of control than the process of generating new proteins from DNA and RNA. The sum of the two processes, including the signals involved in completing them, is called phosphoregulation.
AS FEATURED ON:
AS FEATURED ON:











Discussion Comments
After reading this article, I imagined how proteins works in a single cell. It's really amazing. We are trying to understand the insides of our cells. If we understand completely, it will be the story of us.
@turkay1-- There is actually two sides to disease and protein phosphorylation. Protein phosphorylation can help contain the damage done by disease as well as cause disease itself.
The way it does the first part is that it can destroy cells which have been damaged and cannot function properly. This could be because of disease or something else.
Protein phosphorylation can also contribute to disease if it doesn't work right. This is called abnormal protein phosphorylation. You must have read that protein phosphorylation impacts genes, right? Well, if it doesn't do it's job correctly, it can affect protein expression and cause mutations in our genes.
Scientists think that protein phosphorylation is linked to diseases like cancer, arthritis and diabetes. It's not entirely proven yet because there is the possibility that abnormal protein phosphorylation results from disease rather than being the cause of it. So it's still being studied.
Hope this helps!
@turkay1-- That's right. The main role of protein phosphorylation is to activate protein enzymes. Dephosphorylation works to deactivate them. Protein phosphorylation can deactivate protein enzymes too, but it doesn't happen very often.
When thinking about protein phosphorylation, remember that it is not a rigid process. Phosphorylation will do whatever is necessary at that point in time. It could activate or deactivate proteins, it cause them to bind or break off. We can't say that protein phosphorylation only does one type of activity and the activity can change from protein to protein.
Protein phosphorylation is a regulatory process, so how it acts depends on what is necessary to regulate the cell environment at that point.
There are actually quite a few ways to measure protein phosphorylation. I think most of them do a protein assay, or antibody and kinase assays. They basically analyze samples and check for proteins, protein phosphorylation antibodies or protein kinases. It gives a pretty good idea of how much phosphorylation is taking place and what it is doing.
Thank you for this great article. I was looking for some extra information to help me with my homework and this is helping a lot.
I just need some clarification about phosphorylation and dephosphorylation. Since dephosphorylation undoes the work done by phosphorylation, does this mean that phosphorylation can only activate proteins and dephosphorylation can only deactivate?
The other thing I'm curious about is how disease fits into all of this. Can a virus or bacteria mess up this whole process? Can doctors and scientists do a protein phosphorylation assay to diagnose disease, for example?
Post your comments