What is the Ozone Layer?
The ozone layer is a portion of the Earth’s atmosphere that contains relatively high levels of ozone, O3. The Earth’s atmosphere consists of many different layers and is made up primarily of nitrogen, with oxygen being the second-most common element. The ozone layer is important for a number of reasons, but primarily because it helps to protect life on earth from damaging ultraviolet radiation.
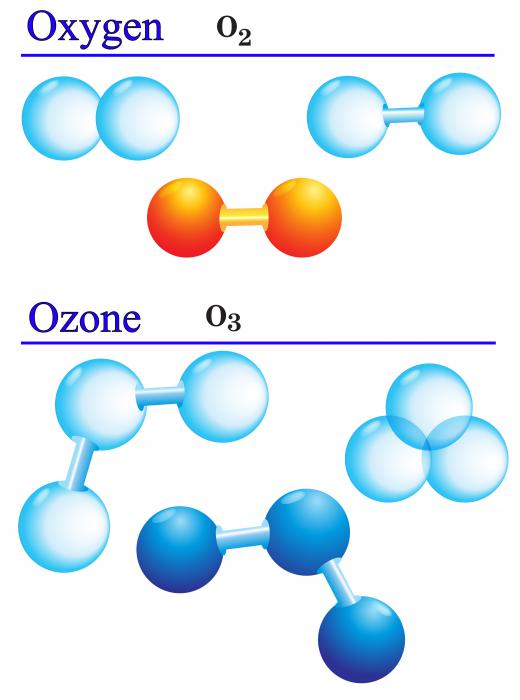
Ozone itself is a particular form of oxygen, where three atoms of the element have bonded together. It is poisonous for humans to breathe directly, and it is considered a pollutant if it’s found near the surface of the Earth. The name comes from the word for the particular smell is it associated with, which occurs during lightning storms.
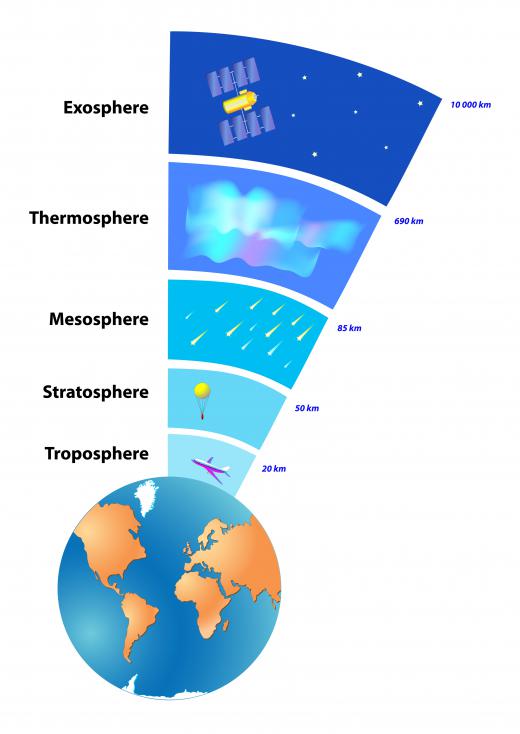
The ozone layer, like the Earth’s atmosphere itself, has no exact boundary. In general, it is viewed as being the layer of gasses 10 to 20 miles (15 to 35 km) above the Earth’s surface. The concentration of ozone in the layer is high in comparison to anywhere else, but it is still relatively low. Even in the most densely-concentrated portions, ozone makes up only a few parts per million.

Ozone is particularly important to humans because it has the unique property of absorbing ultraviolet radiation. There are three main types of ultraviolet (UV) radiation, known as UV-A, UV-B, and UV-C. When functioning properly, this layer of atmosphere completely removes UV-C radiation, which is the most harmful to humans. It also drastically reduces the amount of UV-B that reaches the Earth’s surface — UV-B is the radiation responsible for many types of skin cancer and sunburns.
In the 1970s, it became apparent that the ozone layer was slowly disappearing. It was discovered that this was a direct result of the use of certain catalysts being released in large amounts by humans. A number of countries took small steps to reduce the emission of these catalysts — particularly chlorofluorocarbons (CFCs) — but the steps were generally quite limited. In 1985, however, an enormous hole in the ozone layer was discovered above Antarctica.

The hole provided the necessary impetus for a worldwide movement to help protect this layer of the atmosphere. Within two years of its discovery, the Montreal Protocol was ratified, severely limiting the production of ozone-depleting compounds. By the mid-1990s, the use of ozone-depleting compounds had been drastically reduced, and the ozone layer was on its way to recovery.
Although the atmosphere is still well below its historic levels of ozone, its depletion does seem to have slowed dramatically, and the most immediate danger seems to have passed. The ozone layer serves as a poignant example for many people that the nations of the world are capable of taking relatively quick and concrete action in the face of an impending global catastrophe.
AS FEATURED ON:
AS FEATURED ON:














Discussion Comments
@TreeMan - Unfortunately, you are probably right about no more Montreal Protocols happening. Even the United States won't sign the Kyoto Protocol, which is meant to limit greenhouse gas emissions. Every other country in the UN has signed it.
Admittedly, the global warming problem is much more complex than ozone layer depletion. It was pretty evident what was causing the ozone layer to be destroyed, and there was a simple alternative that was already discovered.
No one is yet positive who or what is causing global warming or how to stop it.
@TreeMan - As you probably guessed, the Montreal Protocol was a meeting held in Montreal that was primarily aimed at the ozone problem. They covered a few other things, but CFCs were the big thing.
They basically came up with a system where countries who signed the agreement would phase out their CFC production in favor of other things that wouldn't react with ozone in the atmosphere.
It was a very interesting system, though. Countries that were already developed were supposed to immediately slow production and get rid of CFCs in a couple of years. Less developed countries like those in Asia were given a bit longer, because they were still developing and would need more time to pay for the new production facilities. At the same time, developed countries gave them loans to help.
As you can image, there was a lot more to it, but those are the basics. It's usually cited as one of the first environmental agreements and is still seen as a huge success.
I never knew what the purpose of the ozone layer was. I had obviously heard of the hole and knew it was bad, but I didn't know why. UV rays are definitely bad, so it's good they discovered the problem and knew what was causing it.
What exactly was the Montreal Protocol, though? It seems very odd that people would be able to get together so quickly and solve a problem like this. Given the hang-ups with the whole global warming controversy, I find it hard to believe that something else like that could ever happen again.
@anon198167 - I always wondered about this as well. It has a lot of factors that play into it.
The start with, the air currents in the upper atmosphere tend to shift things like CFCs toward the poles somehow. I don't know the whole science of it, though.
Another of the reasons is just the temperature. Something about cold air makes the CFCs break apart, so that they can join with the oxygen molecules.
I'm sure a few searches will come up with a much more detailed explanation if you are interested.
Good simple explanation. What I don't understand is why the hole was found over Antarctica. Please explain because you would think the damage would be worse in the area of most pollution.
@calpat -- There are several things you can do, on a personal level, that will help to not destroy the ozone layer further.
One thing you can do, and this is possibly the easiest one, is to avoid using aerosol cans that emit chlorofluorocarbon (CFC).
You can also make sure all of your air conditioning units are working properly, because faulty air conditioners can emit CFC also.
I am so glad I read this article! I remember hearing all about the hole that was found in the ozone layer, but, not knowing much about what that meant, I didn't really pay much attention to it.
Now I understand the importance of the ozone, and I'm going to make sure that I, personally, and doing what I can to preserve it. I'm not sure exactly how to go about it though. Any suggestions?
While the most immediate danger to the ozone appears to have passed, there are two different reasons why continued limitation of the use of ozone-depleting compounds is necessary. For one, scientists are still not entirely sure if global climate change, one of the effects of ozone depletion, is part of a natural cycle; while it might be, that still could mean that the problem may dramatically worsen, since we still do not understand that cycle.
Another reason is that while some technological advances have been achieved that do not use any of these compounds, others use more than any inventions before. With so much still an unknown, it is important to limit in the ways we can.
Post your comments