What are Phosphorylation Sites?
Phosphorylation sites are particular areas of molecules that undergo the addition or removal of a phosphate group known as PO4. They are particularly important for the regulation of proteins in the cell. Phosphorylation can activate or deactivate the protein and is an important way of regulating pathways in the cell. A single protein can have many phosphorylation sites, and each individual cell can have thousands of them. Some results of phosphorylation gone awry can include cancer and diabetes.
Proteins that can be phosphorylated include protein enzymes, which greatly accelerate the speed of reactions. The phosphorylation of a protein can change its function or localization in the cell. Individual enzymes vary in whether their active form has the phosphorylation site phosphorylated or empty.
Receptors are also important sites of phosphorylation. These transmit signals, and signal transduction pathways are often regulated by phosphorylation sites. One of the factors that makes them advantageous for regulation is that the timing of the signaling reactions can vary from hours to less than a second. The pathways of phosphorylation can be highly complex with a series of proteins sequentially phosphorylating the next. This leads to amplification of a pathway.
A protein that adds a phosphate is called a kinase. These regulate a large number of reactions within the cell. Proteins that remove a phosphoryl group are called phosphatases.
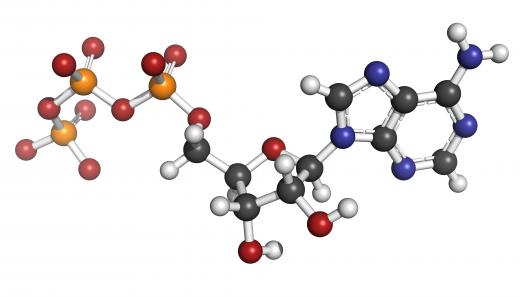
Kinases obtain the phosphate group from adenosine triphosphate (ATP). They add the phosphate group to one of three amino acids — serine, threonine, or tyrosine. Others can act on all three, or even on additional amino acids such as histidine. Some kinases have multiple specificities and can act on more than one target. Such broad target specificity allows the coordinated regulation of multiple pathways by one signal.
One very important subgroup of kinases is the serine/threonine protein kinases. Their phosphorylation site is the OH group of serine or threonine. Phosphorylation by these kinases can be regulated by chemical signals, as well as by events such as DNA damage. MAP kinases are a well-studied group of this type, and a subgroup of MAP kinases is known as extracellular signal-regulated kinases (ERK).
ERK phosphorylation is widely expressed as an intracellular signaling mechanism. What is important about these ERK kinases is that they transmit extracellular signals and amplify them inside the cell. The pathway is activated by many different extracellular factors, including growth factors, hormones, and carcinogens. The ERK pathway is disrupted in many cancers.
Protein phosphorylation, and the location of phosphorylation sites in particular, is a highly active field of study. Up to half of the proteins in a cell can be phosphorylated. Various companies specialize in predicting which areas of a protein can be phosphorylated.
The protein phosphorylation assay generally utilizes antibodies. These are proteins produced by an animal’s immune system that are specific for foreign invaders. There are hundreds of antibodies that are specific for structural changes induced by phosphorylation. Proteins are run on a gel that separates by size and charge, and is known as 2-dimensional electrophoresis. It is then treated with the phospho-specific antibody to determine differences in structure.
It should be noted that other types of molecules can also be phosphorylated. For instance, the phosphorylation of sugars is an important part of cellular metabolism. The energy producing metabolic pathway glycolysis is one such example. The first step in the breakdown of glucose is the phosphorylation of an OH group on the glucose molecule.
The phosphorylation of adenosine diphosphate (ADP) to ATP is essential for many of the cell’s energy-intensive reactions to take place. ATP is a high-energy molecule and gives off energy when it donates a phosphate group. Protein synthesis is among the many important cellular processes powered by ATP.
AS FEATURED ON:
AS FEATURED ON:












Discuss this Article
Post your comments