What is Kelvin?
 Tricia Christensen
Tricia Christensen
The kelvin, (spelled with a lowercase K) is a measurement of heat energy or temperature, which advances in the same increments, as does Celsius. Its principle difference is that kelvin measurements, written as K have a much lower starting point: 0K or 0 Kelvin (note the absence of the degree symbol °). This temperature, measured as -273.15° C, is the point at which no heat energy exists in a substance, and is called absolute zero. To determine the temperature based on kelvins from a Celsius temperature, you merely need to add 273.15 to the Celsius number.
Engineer, physicist, and mathematician, William Thomson, developed the concept of the kelvin in the 19th century. He later was titled Baron Kelvin, after the Kelvin River located near Glasgow University where he had developed the temperature scale. His desire in creating this measurement was to provide a simple way to measure absolute values, particularly absolute zero in a simpler way than was expressed with the Celsius scale.
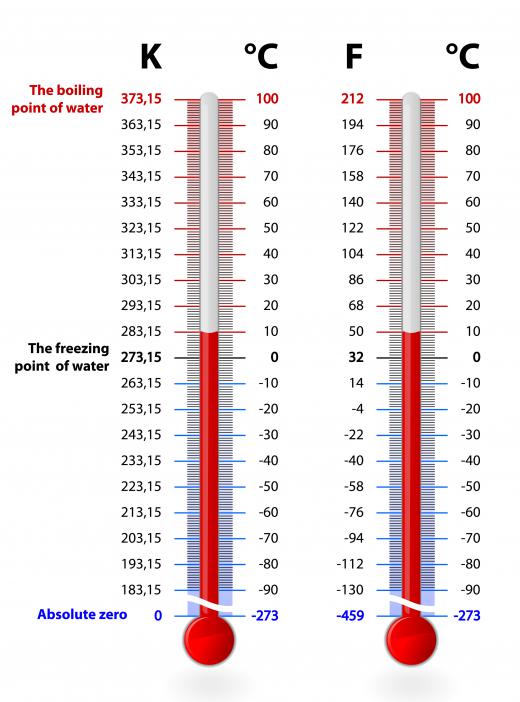
It bears additional mention that kelvins are not measured by degrees. They were considered so until 1968, when the 13th General Conference on Weights and Measures determined to drop the degree reference. This decision was made because Thomson’s measurement referred to an absolute and specific temperature (where no heat energy exists). Celsius, conversely, uses the point of reference of water freezing at the bottom of its scale, and this doesn’t accurately account for heat energy left in the water at this point (273.15 K). Instead, these temperature units are thought of as kelvins. When you measure something by Celsius, for example the boiling point of water, you are measuring in degrees (approximately 100° C). The boiling point of water on Thomson’s scale is approximately 373 kelvins or written as 373 K.

There are some important marking points for Thomson’s scale. Absolute zero is 0 K, and the triple point of water, where water can exist as gas, liquid and solid is 273.16 K (.01° C or 32.018° F). The melting point of ice, 0° C or 32°F, is 273.15 K. The boiling point of water, approximately 100° C or 212° F, is exactly 373.1339 K.
The scientific community often uses kelvin and Celsius measurements interchangeably or at the same time. You may see data on temperature given both a C degrees measurement and a kelvin measurement. This is especially the case when discussing heat energy units between the melting point of ice and absolute zero.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
@ Post 1, kelvin, being absolute, is not affected by atmospheric pressure.
@robbie21 - Yeah, the whole no-degrees thing is kind of weird. The idea, I think, is that a kelvin is a unit of measurement. It is supposed to be sort of on equal footing with the other metric units, like meters, grams, and so forth.
But having one degree Celsius equal one kelvin sure makes conversion easier. And when you get up to really high temperatures, like the surface of the sun, it really doesn't matter whether you use Celsius or kelvin, because you're talking about a difference of a few hundred degrees (or a few hundred kelvins) out of many thousands.
@ Post 1 - You're right. I wonder if that's part of the advantage of kelvin over Celsius. Celsius is still based on water boiling and freezing and these are depending on atmospheric pressure. Absolute zero, on the other hand, doesn't depend on anything--it is the very bottom, as cold as anything could possibly be.
What I don't get is this idea that there are no degree in the kelvin scale. Why not? Especially because a degree kelvin--excuse me, "one kelvin," is exactly equal to a degree Celsius.
This should specify the boiling point of water is 373K at 1 atmosphere of pressure
Post your comments