What is Technetium?
 Mary McMahon
Mary McMahon
Technetium is a metallic chemical element which does not appear naturally, because it has no stable isotope. It bears the distinction of being the first synthetically produced element, after a great deal of experimentation by scientists who predicted its presence on the basis of the order of the periodic table of elements. Consumers generally do not interact with technetium, since it is radioactive, although it is used as a radioactive tracer for some medical tests, so people with certain illnesses may be familiar with it.
In appearance, technetium looks almost like platinum, with a bright, silvery gray color. In moist air, the element will slowly oxidize, and it needs to handled carefully because of its radioactivity. Technetium will also dissolve in certain substances, such as nitric or sulfuric acids. It is identified on the periodic table with the symbol Tc, and it has an atomic number of 43, placing it between molybdenum and ruthenium.

The history of the element is rather complex. The existence of technetium was first hypothesized by Dmitri Mendelev, who noted a gap in the periodic table which he assumed would be filled by an as-yet unknown element. Throughout the 1800s, chemists found a number of substances which they proposed as the missing element, but these turned out to be impure forms of other elements. Finally, in 1937, Carlo Perrier and Emilio Segre produced technetium in their laboratory by bombarding molybdenum in a cyclotron. The new element was named technetium in honor of the technology which facilitated the discovery.
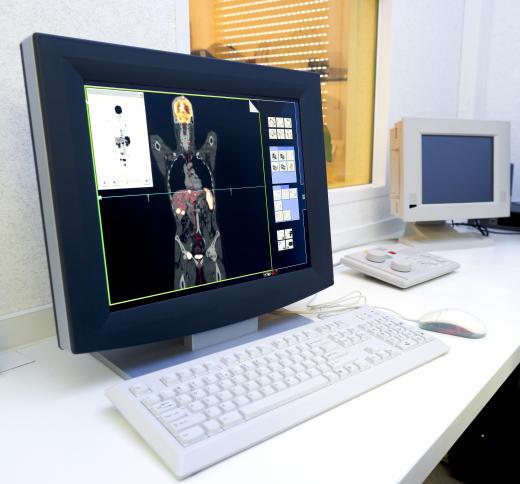
Most of the world's technetium is produced as a byproduct of nuclear fission, and it tends to be expensive. The element is used in chemistry as a catalyst for a variety of reactions, and it is also employed in nuclear medicine. Some scientists also believe that it could be used as an additive in metal alloys to help metals resist corrosion. Access to technetium is generally controlled, since the element is radioactive and therefore potentially dangerous in the hands of people who are inexperienced.
Small amounts of technetium enter the environment through the detonation of nuclear weapons, improper disposal of medical waste, and emissions from nuclear plants. People can absorb the element through air and water, which could cause health problems in high concentrations. Most technetium appears to be expressed by the body, which is why it can be safely used in medical imaging. Specialized testing can be performed to look for technetium exposure in people who may be at higher risk.
AS FEATURED ON:
AS FEATURED ON:












Discussion Comments
I am working on a project about this element and it really helped me. Thanks for the information.
Post your comments